In this project we evaluated the use of a bud of a red cultivar, grafted on individual white cultivar vine stems to serve as visual indicators of leafroll infection in the white cultivar.
Introduction
To control leafroll in vineyards, it is important to plant certified leafroll-free vines to control mealybug numbers and their spread, and to annually rogue infected plants. When applied stringently, these strategies have been found to be very effective in reducing the number of leafroll-infected vines to very low levels (Figure 1) and thereby prolonging the economic lifespan of the vineyard.

FIGURE 1. View of red-cultivar vineyards in autumn on a wine estate where leafroll control is applied stringently. Notice the absence of any vines displaying leafroll symptoms.
Identifying infected vines in relatively healthy vineyards of most red wine cultivars is easy as symptoms are obvious in late summer and autumn allowing these infected vines to be marked annually for removal in winter (Figure 2). For at least four of these cultivars (Cabernet Sauvignon, Shiraz, Merlot and Cabernet franc), the visual detection of the disease has been shown to correlate extremely well with ELISA tests for grapevine leafroll-associated virus 3 (GLRaV-3), the major cause of leafroll in South Africa.

FIGURE 2. Obvious symptoms of leafroll infection in autumn within a red cultivar vineyard where leafroll control is stringently applied. Infected vine marked with white PVA paint for removal (roguing) in winter.
White cultivars do not show clear leafroll symptoms (Figure 3) and to control the disease by roguing laboratory-based tests must be done annually for GLRaV-3.

FIGURE 3. Leafroll symptoms in autumn on Chardonnay, one of the few white cultivars that shows some leafroll symptoms, albeit these are only expressed following a relatively long latent phase in which no symptoms are observed. Some cultivars, such as Sauvignon blanc, never show any obvious symptoms when infected by grapevine leafroll-associated virus 3 (GLRav-3) infection, but are negatively affected by the disease.
This makes control of the disease on white cultivars extremely expensive and difficult to achieve with the large numbers of vineyards planted to white cultivars. Should the vine become infected with GLRaV-3, the disease will manifest itself in the so-called “sentinel cane” (the red-grafted shoot) of the vine, making visual assessment for the disease possible (Figure 4).

FIGURE 4. Use of a sentinel cane to express leafroll disease on a white-wine cultivar. Berries retained on the sentinel cane are purely for illustrative value and would have been removed in practice.
The sentinel cane need only be grafted onto the vine once in its lifetime and can be pruned back annually. This would circumvent the need to do laboratory tests yearly and makes roguing of infected vines cheaper and more practical. We foresee that this technique could be used primarily in white cultivar foundation and mother-blocks of the One Vitis certification scheme, but also at growers who wish to achieve low or zero leafroll infections on their estates and therefore also control leafroll in the white cultivars on the estate.
Method
In a field trial conducted from 2015 to 2019, we tested the relative sensitivity of Pinot noir or Cabernet franc as sentinel cane indicators on various white cultivars (mainly Chenin blanc) to detect leafroll compared to ELISA detection of GLRaV-3 in green-berried vines. We tested grafting of the sentinel cane bud onto the stem through three grafting techniques (chip-budding, omega sandwich graft and amphi-grafting (Figure 5).
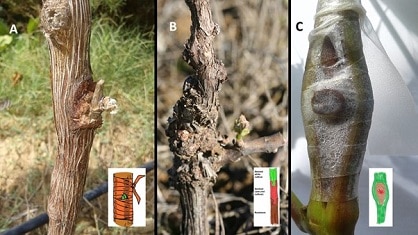
FIGURE 5. Three methods utilised for the grafting of the red cultivar sentinel buds onto the white cultivar stems. A) Chip-budding, B) omega sandwich graft of stem, and C) amphi-grafting (dormant winter bud grafted onto green stem).
We tested the ability of the sentinel cane to detect grapevine leafroll-associated virus (GLRaV-3), the most prevalent cause of leafroll disease in South Africa, by artificially inoculating the sentinel cane-grafted vines with GLRaV-3 through either graft inoculation of GLRaV-3 infected stem pieces (Figure 6A), or by placing mealybugs which had been feeding on GLRaV-3 infected vines onto leaves of the sentinel cane grafted vines (Figure 6B).

FIGURE 6. Inoculation of white wine cultivar scion with grapevine leafroll-associated virus 3 (GLRaV-3) using A) amphi-grafting of GLRaV-3 infected stem pieces, or B) of Planococcus ficus mealybugs feed on GLRaV-3 infected vines and placed on the healthy plant in insect cages.
Using only chip-budding, we attempted to test the graft compatibility of Pinot noir or Cabernet franc onto the most common wine grape cultivars, as well as their relative usefulness to manifest GLRaV-3 symptoms as sentinel canes (Figure 7).
FIGURE 7. Chip-budding of either Pinot noir or Cabernet franc sentinel canes on various white wines to assess the graft compatibility and usefulness of these two cultivars as sentinel canes.
We also tested the placement of the sentinel cane relative to the GLRaV-3 inoculation site on the ability of the sentinel cane to detect GLRaV-3, and the potential effect of the systemic spread through various rootstocks (Figure 8).
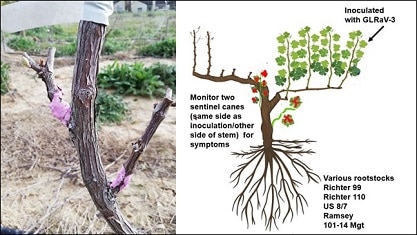
FIGURE 8. Chip-budding of sentinel canes on either side of the stem, with inoculation on one cordon and various rootstock cultivars, to determine whether there is a difference in time of leafroll expression on the two sentinel canes, because of the sentinel cane placement and systemic movement of GLRaV-3 through different rootstocks.
Results
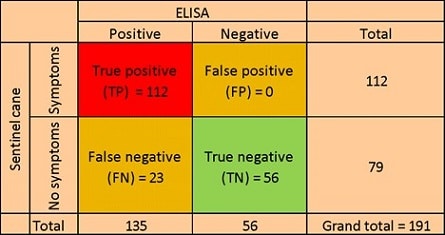
Sentinel canes have a sensitivity (ability to correctly test those with the disease), specificity (ability to correctly identify those without the disease), and accuracy (percentage of true positives and true negatives identified within the population) of 82%, 100% and 87% respectively relative to ELISA (Table 1).
TABLE 1. Analysis of the sensitivity and specificity of using sentinel canes (results of Pinot noir and Cabernet franc combined, n = 191) relative to ELISA for the detection of grapevine leafroll-associated virus 3 (GLRaV-3). Sensitivity (ability to test correctly those with the disease) = TP/(TP + FN) x 100 = 82%. Specificity (ability to correctly identify those without the disease) = TN/(FP + TN) = 100%. Accuracy = (TP + TN)/total population = 87%.
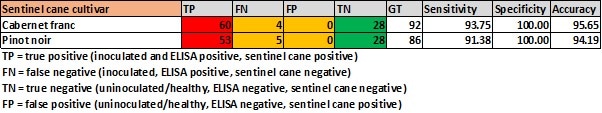
As expected from such a high sensitivity and specificity, very little differences were observed regarding the reliability of using either Pinot noir or Cabernet franc as sentinel canes, and either can be used (Table 2).
TABLE 2. Sensitivity, specificity and accuracy determination of two cultivars used as sentinel canes relative to ELISA results.
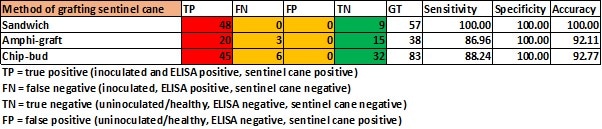
The sensitivity and specificity of the three means of grafting the sentinel cane is presented in Table 3. With all three methods providing a very high correlation with ELISA test for GLRaV-3. Chip-budding, however, proved to be the method with the least take, and comparative numbers with the other grafting methods were only obtained, because in the experiments many more chip buds had been conducted than the other two methods. Chip-budding could only be conducted in the field in the second season of growth as the stem diameters of the white cultivars were too thin in the first season. When done in the second growth season, take on the stems was low with less than 50% of the grafts sprouting. All chip-bud grafts (n = 151) that had not taken by the second season were re-grafted in the third season, but very few took, and it is clear this method of grafting onto established vines is problematic and probably not practical once vine apical dominance has been established.
TABLE 3. Sensitivity, specificity and accuracy determination of means of sentinel canes grafted with three different methods relative to ELISA results.
Sandwich-grafting in the nursery was a very efficient and reliable means of grafting the sentinel cane with 80 of 105 (76,19%) successful graft takes across all scion cultivars and rootstocks and with both Pinot noir and Cabernet franc utilised (Figure 9). The position of the sentinel cane in the fully grown vine, however, was too close to the ground. It may be worth assessing whether longer inter-stocks of the sentinel cultivar can be used to overcome this. The sentinel cane shoot derived from the sandwich grafts were also relatively vigorous and required considerable post-graft management.

FIGURE 9. Sandwich graft take over various Vitis cultivars and Richter 110 rootstock.
Amphi-grafts also yield very high take of the bud, but can only be performed while the stem is still not lignified. This would have to be done in the first or second season of growth in the vineyard, or stems would have to be cut back and allowed to re-sprout otherwise. This method of grafting of the sentinel cane once vines are already established is preferred to chip-budding.
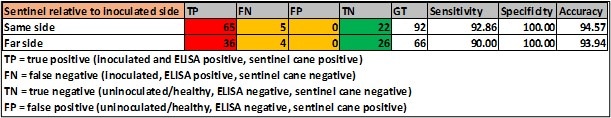
A comparison with the detection of GLRaV-3 in the upper canopy and sentinel canes (obtained through any grafting means) either on the same side of the stem as the inoculation site in the cordon or on the opposite site is provided below (Table 4).
TABLE 4. Detection of GLRaV-3 by sentinel canes; position of sentinel cane relative to site of inoculation.
Conclusions
Sentinel canes have proven to be only slightly less reliable than ELISA for the detection of grapevine leafroll-associated virus 3 (GLRaV-3) infection. Sensitivity (ability to test correctly those with the disease), specificity (ability to correctly identify those without the disease), and accuracy (percentage of true positives and true negatives identified within the population) of 82%, 100% and 87% respectively were found for the sentinel canes relative to ELISA. In general, the only difference being that sentinel canes yielded false negatives on a few occasions, not showing symptoms when the canopy tested positive for GLRaV-3. It is possible that had the symptoms been recorded later in the season, some of those sentinel canes may have expressed symptoms. As with visual monitoring leafroll symptoms directly on red cultivars, some vines only display symptoms later in the season. No significant differences were observed regarding the reliability of using Pinot noir or Cabernet franc as sentinel canes, or whether the sentinel cane had been established through sandwich grafts, chip-budding or amphi-grafts. However, poor graft-take using chip-budding would discount this means of grafting in commercial settings. While the sandwich grafts have a very good take and can be prepared in the nursery, they result in sentinel canes that are too low on the stem, are too vigorous and possibly also affect the vigour of the canopy. Amphi-grafts by contrast, are easy to perform and have an excellent rate of take, but would require in field-grafting, preferably within the first season while the stem is still a green shoot. To achieve this, buds would have to be taken from thinner dormant canes.
Due to the poor take of chip-budding, the experiment to determine whether any genetic incompatibility of Pinot noir and Cabernet franc exists when grafted onto the most prevalent white cultivars in South Africa did not yield sufficient replicates to determine if small differences in take exist. However, comparable sandwich grafts with Chenin blanc, Sauvignon blanc, Chardonnay, Semillon and Colombar did not show any obvious incompatibilities.
As rootstocks do not display leafroll symptoms and GLRaV-3 is poorly detected in them, it is possible that rootstocks may affect the spread of the virus in the vine itself. However, very little difference was observed in detection of GLRaV-3 by sentinel canes relative to ELISA when grafted on the stem on the same or opposite sides to the cordon to the site of inoculation.
Take home message
The project suggests that sentinel canes are a feasible means of detecting GLRaV-3 infection in white cultivars and that it has potential for use in the certification scheme, possibly in white cultivar foundation vineyards to identify infected vines for annual roguing. The technique also has potential use for commercial wine estates, serious about controlling leafroll disease.
– For more information, contact Gerhard Pietersen at Patho Solutions – gerhard@pathsol.co.za.













